6.12: Second Law for a Refrigration Cycle#
Problem Statement#
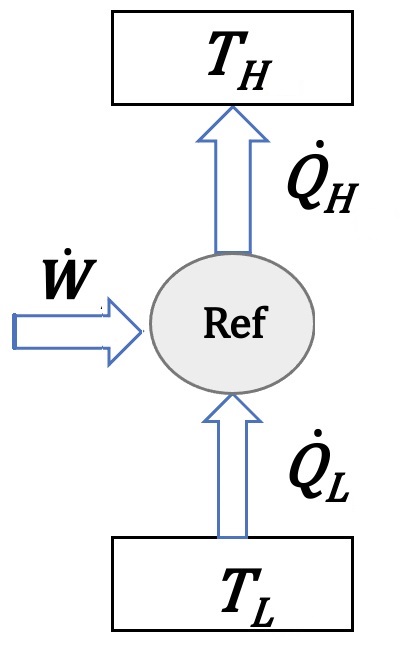
A refrigration cycle works between a cold space at temperature \(T_L\) and a heat sink at a high temperature \(T_H\). Given the cooling load of the cycle is \(\dot Q_L=50\:kW\) and the compressor consumes \(\dot W=10\:kW\) of electric power, calculate:
a) heat dissipated into heat sink
b) COP of the cycle
Assuming the heat absorbtion from the cold environment happens at \(T_L=-20\:^{\circ}C\) and the heat dissipation to the heat sink is at \(T_H=60\:^{\circ}C\), calculate
c) entropy generation (is this feasible?)
d) COP of the Carnot cycle (how does this compare?)
Assuming the cycle operates with a Carnot COP,
e) how much work is required for the same cooling load
f) how much heat is dissipated into the heat sink
g) entropy generation
Solution Approach for a)#
from the first law
\(\dot Q_H=\dot Q_L+\dot W\)
#define inputs
Q_L = 50e+3 #heat absorbed from cool space in W
W = 10e+3 #cycle work input in W
Q_H = Q_L + W #heat dissipated into heat sink in W
print('The amount of heat dissipated into heat sink is:', f"{Q_H/1000:.1f}", 'kW')
The amount of heat dissipated into heat sink is: 60.0 kW
Solution Approach for b)#
for a refrigration cycle
\(COP=\dot Q_L/\dot W\)
COP = Q_L / W #COP of the cycle
print('The COP of the cycle is:', f"{COP:.1f}")
The COP of the cycle is: 5.0
Solution Approach for c)#
from the second law for a closed system
\(S_2-S_1=\Sigma Q/T+S_{gen}\)
since the cycle is steady
\(S_2-S_1=0\)
therfore
\(S_{gen}=-\Sigma Q/T=-(Q_L/T_L-Q_H/T_H)\)
#define variables
T_L = -20 + 273.15 #low temperature in K
T_H = 60 + 273.15 #high temperature in K
S_gen = -1 * (Q_L / T_L - Q_H / T_H) #negative sign for Q_H due to heat leaving the system
print('The entropy generation of the system is:', f"{S_gen:.1f}", 'W/K')
The entropy generation of the system is: -17.4 W/K
Solution Approach for d)#
\(COP_{Carnot,Refrigration}=1/(T_H/T_L-1)\)
COP_C = 1 / (T_H / T_L - 1)
print('The Carnot COP for the cycle is:', f"{COP_C:.1f}")
The Carnot COP for the cycle is: 3.2
Solution Approach for e)#
\(\dot W=\dot Q_L/COP\)
W = Q_L / COP_C
print('The work input for the refrigration cycle based on Carnot operation is:', f"{W/1000:.1f}", 'kW')
The work input for the refrigration cycle based on Carnot operation is: 15.8 kW
Solution Approach for f)#
from the first law
\(\dot Q_H=\dot Q_L+\dot W\)
Q_H = Q_L + W
print('The heat dissipation based on Carnot operation is:', f"{Q_H/1000:.1f}", 'kW')
The heat dissipation based on Carnot operation is: 65.8 kW
Solution Approach for g)#
\(S_{gen}=-\Sigma Q/T=-(Q_L/T_L-Q_H/T_H)\)
S_gen = -1 * (Q_L / T_L - Q_H / T_H) #negative sign for Q_H due to heat leaving the system
print('The entropy generation of the system based on Carnot COP is:', f"{S_gen:.1f}", 'W/K')
The entropy generation of the system based on Carnot COP is: -0.0 W/K
