2.10: Water in Two Tanks#
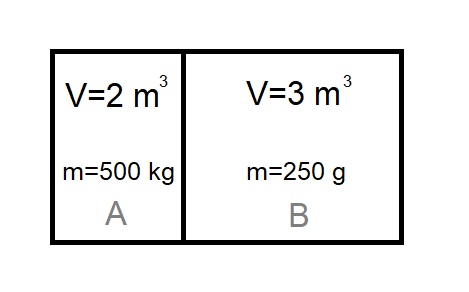
A rigid tank containig water in two seperate rigid compartments (A & B) is shown below. The compartment A contains \(m_a=500\:kg\) of water and occupies \(V=2\:m^3\) of space. The compartment B contains \(m_b=250\:g\) of water and occupies \(V=3\:m^3\) of space. The tank including its compartments is in a thermal equilibrim with its surrounding at \(T=40\:^{\circ}C\). The dicider is then suddenly removed allowing water in two compartments being mixed together. Determine,
a) the liquid mass and pressure of each compartment before mixing
b) the quality and pressure of mixed water
Solution Approach for a)#
the quality of a saturated mixture is defined as
\(x=m_g/m_{mix}\)
therfore the mass of liquid would be
\(m_l=(1-x)m_{mix}\)
#import librarier
import CoolProp.CoolProp as CP
#define variables
V_A = 2 #tank A volume in m3
V_B = 3 #tank B volume in m3
m_A = 500 #fluid in tank A mass in kg
m_B = 0.25 #fluid in tank B mass in kg
T = 25 + 273.15 #temperature in K
D_A = m_A / V_A #density of tank A in kg/m3 used in CoolProp
D_B = m_B / V_B #density of tank B in kg/m3 used in CoolProp
fluid = "water" # define the fluid or material of interest
x_A = CP.PropsSI("Q", "T", T, "D", D_A , fluid) #mixture quality in A
x_B = CP.PropsSI("Q", "T", T, "D", D_B , fluid) #mixture quality in B
m_l_A = (1 - x_A) * m_A #liquid mass in A in kg
m_l_B = (1 - x_B) * m_B #liquid mass in B in kg
print('liquid mass in A is', f"{m_l_A:.2f}", 'kg')
print('liquid mass in B is', f"{m_l_B:.2f}", 'kg')
P_A = CP.PropsSI("P", "T", T, "D", D_A , fluid) #pressure in A in Pa
P_B = CP.PropsSI("P", "T", T, "D", D_B , fluid) #pressure in B in Pa
print('pressure in A is', f"{P_A:.2f}", 'Pa')
print('pressure in B is', f"{P_B:.2f}", 'Pa')
liquid mass in A is 499.97 kg
liquid mass in B is 0.18 kg
pressure in A is 3169.93 Pa
pressure in B is 3169.93 Pa
Solution Approach for b)#
after mixing the total volume and mass would be
\(V=V_A+V_B\)
\(m=m_A+m_B\)
and the temperature remains constant since the tank is in equilibrium with its surrounding
V = V_A + V_B #total volume in m3
m = m_A + m_B #total mass in kg
D = m / V #density of the mixture
x = CP.PropsSI("Q", "T", T, "D", D, fluid) #mixture quality
m_l = (1 - x) * m #liquid mass in kg
print('liquid mass after mixing is', f"{m_l:.2f}", 'kg')
P = CP.PropsSI("P", "T", T, "D", D, fluid) #pressure after mixing
print('pressure after mixing is', f"{P:.2f}", 'Pa')
liquid mass after mixing is 500.15 kg
pressure after mixing is 3169.93 Pa
